From smartphones to electric vehicles and renewable energy storage, batteries are the silent enablers of our daily lives. Their importance will only grow: by 2030, global demand for lithium-ion batteries (LIBs) is expected to grow more than fivefold, driven primarily by EV adoption and grid-scale energy storage solutions (IEA, 2023).
But what powers the battery, and more importantly — what powers its materials?
What Are the Main Types of Batteries Today?
- Lithium-Ion Batteries (LIBs):
Currently the most common rechargeable battery type in use, LIBs power electric vehicles, portable electronics, and renewable energy storage systems. Their advantages include high energy density, low self-discharge, and long cycle life. - Emerging Alternatives:
- Sodium-Ion (Na-ion): Uses sodium instead of lithium — more abundant and cheaper, but with lower energy density.
- Magnesium-Ion (Mg-ion) and other multivalent chemistries: Still under development, these aim to deliver higher capacities with lower cost.
The Anatomy of a Battery: The Anode and Cathode
Every battery contains two key electrodes:
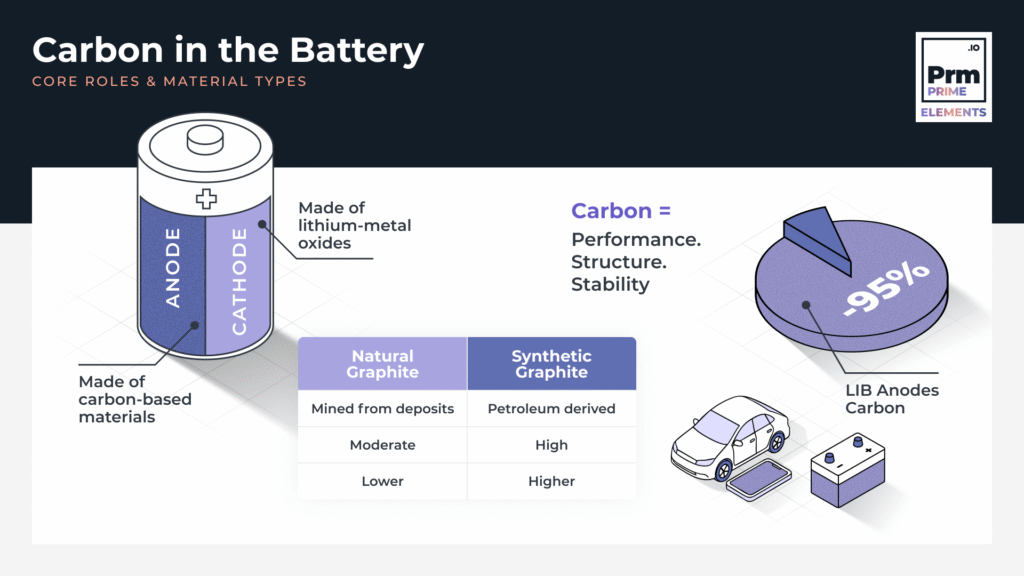
- Cathode (positive electrode): Usually made of lithium-metal oxides (e.g. NMC, LFP), this stores lithium ions during discharge.
- Anode (negative electrode): This is where carbon-based materials come into play. The anode hosts lithium ions during charging and releases them during discharge.
Why Carbon Is Essential to Anodes
Carbon — especially graphitic carbon — is the dominant material used in anodes for modern lithium-ion batteries. Why?
- Electrical conductivity: Carbon provides excellent electron flow.
- Structural stability: It can intercalate lithium ions between graphene layers without degradation.
- Cost and scalability: Natural and synthetic graphite are already available at commercial scale.
As of 2022, over 95% of commercial LIBs use graphite-based anodes (USGS, 2023).
Types of Carbon in Batteries
- Natural Graphite: Mined from deposits, purified, and shaped into anode-grade particles.
- Synthetic Graphite: Produced from calcined petroleum coke (CPC) through high-temperature treatment (up to 3000°C). It offers more uniformity and higher purity.
- Hard Carbon & Soft Carbon: Used in sodium-ion batteries; hard carbon, in particular, shows promise for long cycle life.
Raw Materials: Where Do They Come From?
The two main carbon sources in battery anodes:
- Graphite: China dominates production (~65% of global supply), but diversification is accelerating.
- Petroleum Coke (Petcoke): A byproduct of oil refining, petcoke is a feedstock for synthetic graphite. The calcination and graphitization processes make it suitable for battery-grade carbon.
Are There Alternatives to Carbon Anodes?
While many research efforts explore silicon, lithium metal, or solid-state anodes, these materials face:
- Safety concerns
- Low cycle stability
- High cost
For now, carbon remains irreplaceable for commercial-scale, high-performance batteries.
Conclusion
Carbon isn’t just a “black filler” — it’s a high-performance, engineered material central to the battery revolution. From natural graphite to petroleum-derived synthetic graphite, the anode’s carbon base is the foundation upon which next-generation batteries are being built.
In the next post, we’ll take a closer look at how battery anodes are made — from raw petcoke to precision-shaped graphite particles.
Need carbon materials for battery production?
Contact Prime Elements — your partner for sustainable, high-purity carbon solutions.