Behind every high-performance lithium-ion battery (LIB) is a carefully engineered anode made of carbon. But how exactly is this material produced — and what role do petroleum byproducts play in its journey from raw feedstock to finished anode?
This post takes you behind the scenes of the anode production process, spotlighting key materials like calcined petroleum coke (CPC) and carbon fillers, and how they’re transformed into battery-grade graphite.
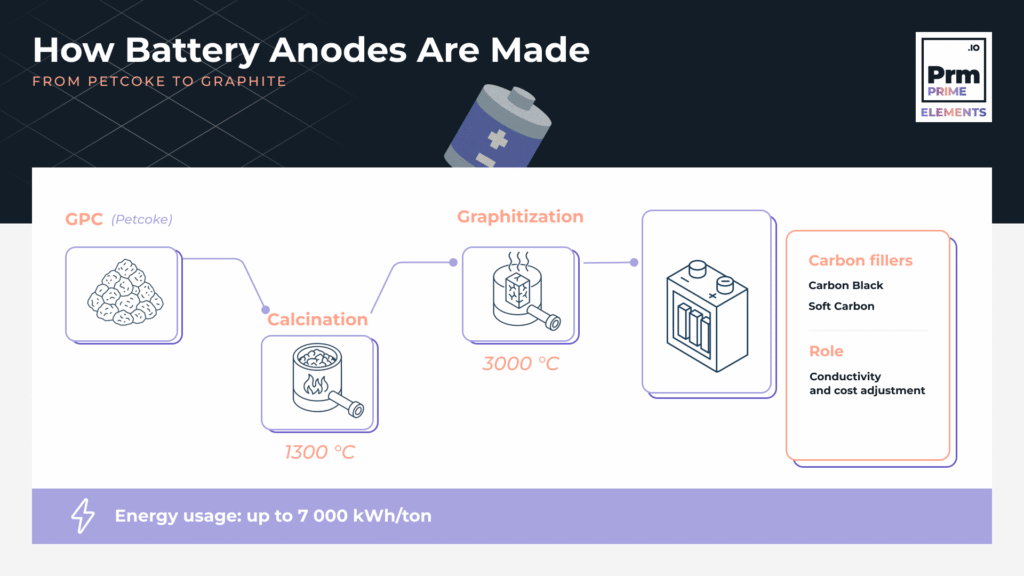
Step 1: Feedstock Selection — Why Petroleum Coke?
Green Petroleum Coke (GPC) is a carbon-rich solid byproduct from oil refining. It contains high levels of carbon (~85–90%) and comes in two main forms:
- Fuel-grade petcoke: High in sulfur/metal content — used for energy
- Anode-grade petcoke: Lower in impurities — used to produce synthetic graphite
To be useful for batteries, GPC must be calcined to remove volatile matter and increase purity.
Step 2: Calcination — From Green to Calcined
In rotary kilns or shaft kilns, GPC is heated to ~1300–1400°C. This removes volatile compounds and enhances:
- Carbon purity (typically >99%)
- Crystallinity
- Electrical conductivity
The result: Calcined Petroleum Coke (CPC) — a purified carbon source ready for further graphitization.
Step 3: Graphitization — Aligning the Carbon Structure
CPC is next treated at temperatures up to 3000°C in electric furnaces. This process rearranges the carbon atoms into highly crystalline graphite structures — ideal for lithium intercalation in battery anodes.
Key properties achieved:
- Low surface area (reduces side reactions)
- High structural order (enhances cycle life)
- Low impurity levels (improves safety)
Step 4: Milling, Shaping & Spheronization
Graphitized carbon is then:
- Milled to achieve consistent particle size
- Shaped/spheronized to improve packing density
- Surface-treated for improved electrolyte compatibility
These steps ensure optimal electrochemical performance, especially for fast-charging EV applications.
Step 5: The Role of Carbon Fillers
In some anode formulations, carbon fillers (e.g., carbon black, soft carbon) are added to:
- Enhance electronic conductivity
- Improve binder interaction
- Lower cost
The choice of filler affects performance, especially in blended systems (e.g. graphite + silicon).
What About Natural Graphite?
Natural graphite undergoes purification
(chemical or thermal) and similar shaping steps. However, it typically offers:
- Lower cost and lower
environmental impact (if sourced sustainably) - Slightly lower uniformity compared
to synthetic graphite
The choice depends on application:
synthetic graphite is still preferred for high-end EV batteries
requiring long cycle life and consistent behavior.
Energy Intensity & Environmental
Footprint
Graphitization is extremely
energy-intensive. Producing 1 ton of synthetic graphite can consume up to
7,000 kWh of electricity. As a result, the carbon footprint and power
source (renewable vs coal-based) are major concerns.
This is driving interest in:
- Low-carbon petcoke
- Waste heat recovery at calcination sites
- Recycled graphite from battery scrap (a topic we’ll cover in Part 3)
Conclusion
From oil refinery waste to high-tech energy
storage — the transformation of petroleum coke into battery anodes is one of
the most overlooked success stories in the energy transition.
At every step, precision, purity, and
carbon control are key. As demand for EVs and stationary batteries soars, so
does the need for high-performance carbon anode materials.
Looking for secure, consistent
feedstocks for battery-grade carbon?
Contact Prime Elements — your trusted supplier for synthetic graphite
raw materials.